Charting a path to breakthrough medicines
Regor combines a cutting-edge drug discovery engine with target validation and optimization to produce stellar preclinical candidates for metabolism, oncology, and immunology.
Rapid validation Proprietary discovery platform Track record of successful partnership
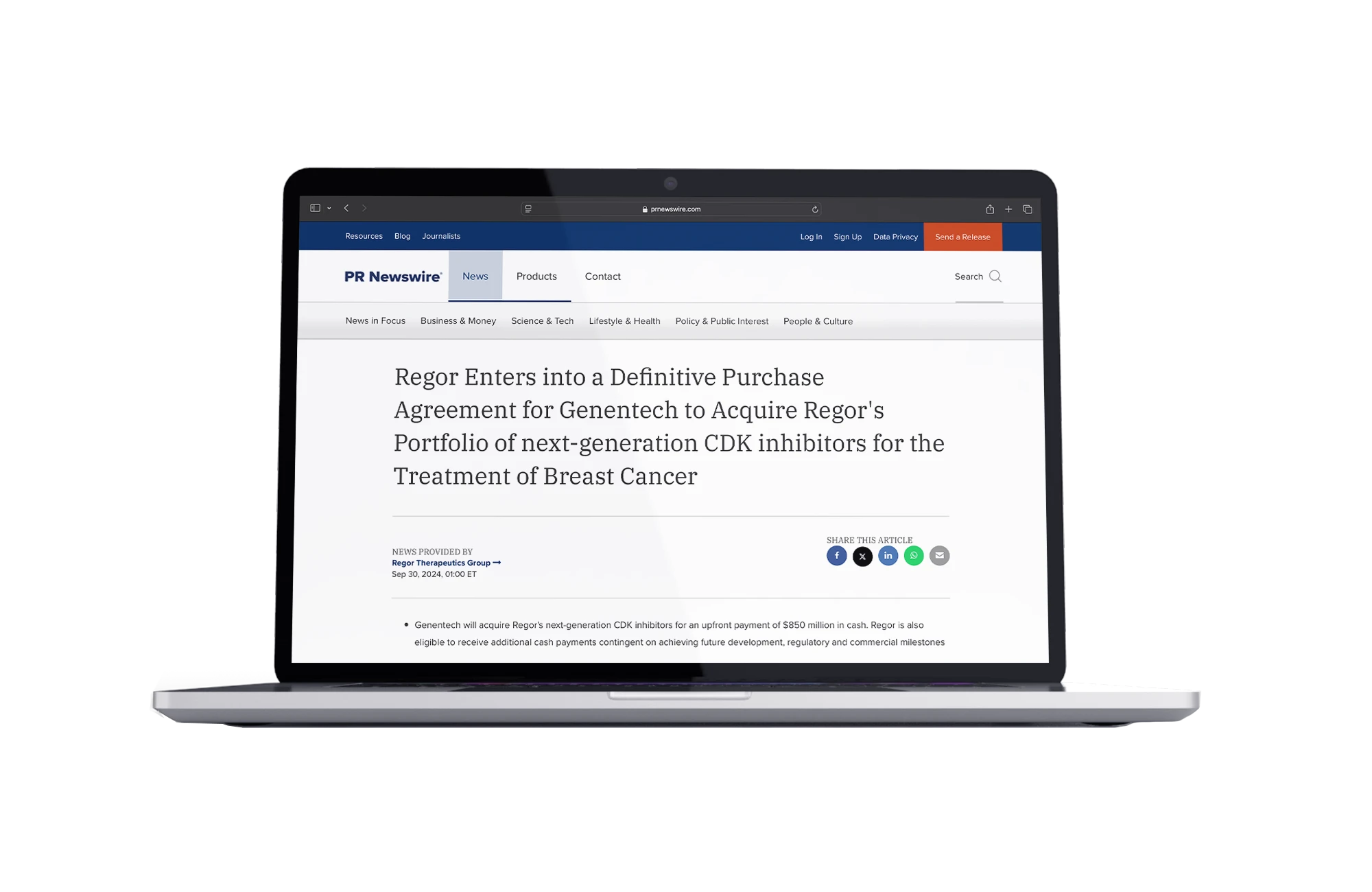
Regor Enters into a Definitive Purchase Agreement for Genentech to Acquire Regor’s Portfolio of Next-Generation CDK Inhibitors for the Treatment of Breast Cancer
- GDC-4198 (RGT-419B) and GDC-0587 (RGT-587)
- Next-gen CDK inhibitors for breast cancer
- Licensed by Roche/Genetech
- Phase-I Trials in progress
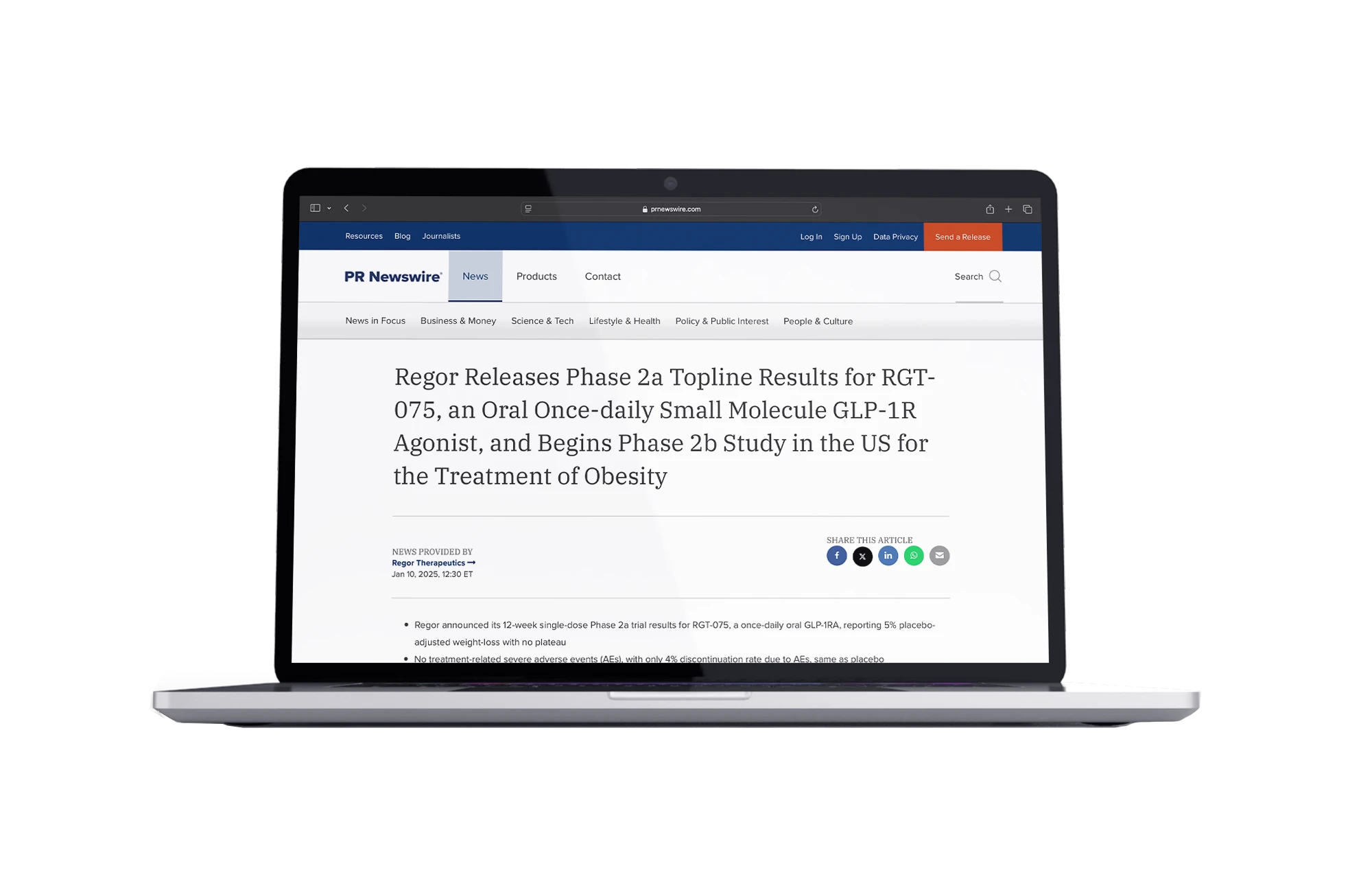
Regor Releases Phase 2a Topline Results for RGT-075, an Oral Once-daily Small Molecule GLP-1R Agonist, and Begins Phase 2b Study in the US for the Treatment of Obesity
- RGT-075
- Leading once-daily oral SM GLP-1R agonist
- Peptide-like efficacy and tolerability
- Phase-II Trials in progress
Meteoric trajectory
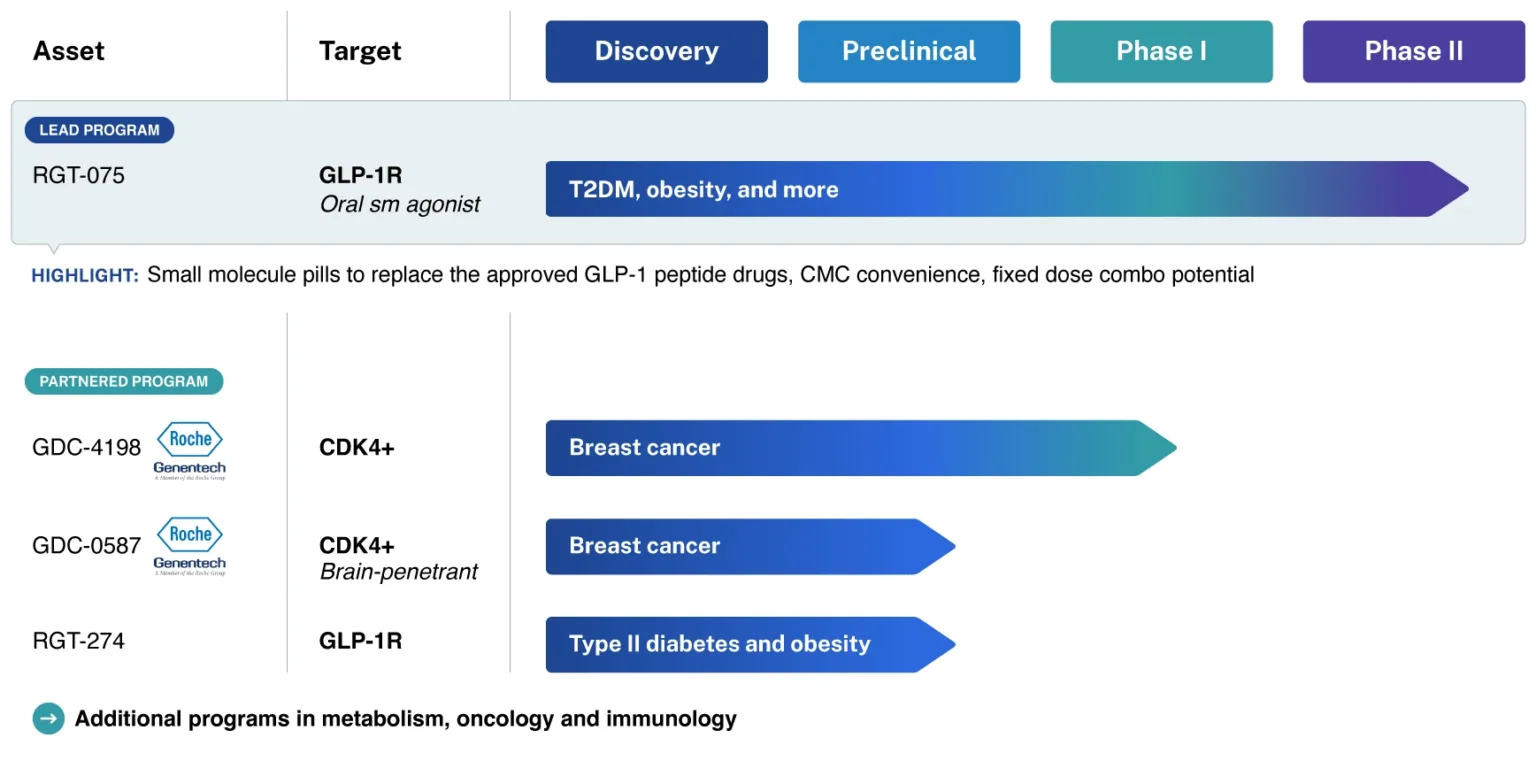
Projects
PCCs
Clinical data assets
Out-licenses
Illuminating technology
Regor’s cutting-edge drug discovery system leverages rCARD (computer-accelerated rational discovery) to discover promising pre-clinical candidates.
Via strategic collaborations and in-house expertise, those candidates are highlighted and optimized before licensing to top pharma for commercial development.

Computational Biology
Target ID Clinical Development
Structural Biology
Target Validation Molecule Design
Computational Chemistry
Molecule Design Optimization
Founded by seasoned drug hunters - named inventors of 4 FDA approved drugs
Collectively 100+ INDs and 50+ NDAs

Domenick Bertelli
Senior Biopharmaceutical Industry Advisor

Jake Bauer
Senior Biotech Advisor

Jeff Capello
Managing Partner, Monomoy Advisor

Julie Wright
Human Resource Advisor

Michael Nauck
Head of Clinical Research at the Diabetes Division of St. Josef-Hospital
(Ruhr-University Bochum) in Bochum, Germany

Zan Fleming, MD
CEO & Co-Founder, Kinexum
Press releases and news
PR Newswire
Jun 05, 2024
Publications of Note

Structural insights into the activation of GLP-1R by a small molecule agonist
Cell Research
doi: 10.1038/s41422-020-0384-8

Molecular insights into ago-allosteric modulation of the human glucagon-like peptide-1 receptor
Nature Communications
doi: 10.1038/s41467-021-24058-z

Accelerated Discovery of Macrocyclic CDK2 Inhibitor QR-6401 by Generative Models and Structure-Based Drug Design
ACS Medicinal Chemistry Letters
doi: 10.1021/acsmedchemlett.2c00515

Direct Construction of Chiral Ether via Highly Efficient Heck Reaction and N‑Directed Asymmetric Hydrogenation for Large-Scale Synthesis of MALT1 Inhibitor RGT-068A
Organic Process Research & Development
doi: 10.1021/acs.oprd.2c00229

A Phase 1, Double-Blind, Placebo-Controlled Multiple Escalating Dose Study of RGT-075, a Novel Small-Molecule Oral GLP-1 Receptor Agonist in Adults with Type 2 Diabetes
Regor Pharmaceuticals

A First-in-Human Study of RGT-075, a Novel, Orally Bioavailable, Small-Molecule GLP-1 Receptor Agonist, in Healthy Adult Subjects
Regor Pharmaceuticals
Stars of hope for patients
Regor is one of the brightest star systems, containing hundreds of invisible protostars that are poised to blow up and reveal themselves. Like protostars, blockbuster pre-clinical candidates are a flash away from clinical significance.
Regor helps find and reveal these protodrugs. Get involved before the next one flares up.

